Application process
The NBB has an open access policy and annually receives and reviews 150-200 requests from academic researchers and pharmaceutical companies from all over the world. The NBB procedures to ensure an effective organization of the tissue transfer process are explained here.
Apply for tissue
Preparing the request for samples
The NBB’s extensive stock of human CNS tissue samples are registered in a members-only online database: the e-NBB. New members can request an account and after approval they can search the collection and make and save a selection of brain samples.
If assistance is required for selecting samples, please contact the NBB via eNBB@nin.knaw.nl. The personnel of the NBB may be able to provide useful advice and expertise on the use of human tissue and tissue availability.
Submission and review of the request
You can request samples through our online database e-NBB. Click on the 'submit application' button on the homepage of e-NBB, and provide all information regarding your tissue request in the subsequent online form.
All applications are reviewed by the NBB’s scientific committee that will review the availability of the requested material (diagnosis and anatomical area) and scientific quality of the project. Within 6 weeks upon receipt of the application form, we aim to inform you whether your request can be approved. The document linked below describes the review criteria for applications.
Eligibility
The NBB applies the following criteria to assess whether applicants and institutions are eligible to request tissues:
- Applicant: must be an employee of a research and/or academic institution, pharmaceutical industry or contract research organization with proven research knowledge.
- Institution: must be a research and/or academic institution, pharmaceutical company or contract research organization. In principle, the NBB does not provide material to institutions that are under military authority or have a clear military signature.
If you have questions regarding eligibility, please contact us via enbb@nin.knaw.nl.
Financial contribution
Cost-recovery
The NBB is a non-profit organization and the financial contribution is meant as compensation for the costs of brain banking, and not as payment for the brain tissue samples as such. With the financial contribution of researchers NBB covers part of the costs for donor recruitment, autopsies around the clock, processing samples in the lab, determining the neuropathological diagnosis, and storage and distribution of samples. When submitting grants for research projects which plan to use NBB tissue, we recommend to include the estimated financial contribution in the grant application budget.
Sample price
The financial contribution per sample for non-profit organizations per 1 January 2024
|
Tissue block formalin-fixed paraffin-embedded (~1cm2) |
€ 83,00 |
|
Tissue block frozen (~1cm2) |
€ 83,00 |
|
Tissue block fresh in medium |
€ 83,00 |
|
1000 μl of csf (~ 1 mL) |
€ 41,50 |
|
Blood plasma (~1 mL) |
€ 41,50 |
|
DNA |
€ 41,50 |
|
10x glass slide-mounted serial FFPE sections |
€ 83,00 |
|
5x glass slide-mounted serial cryo sections |
€ 83,00 |
|
5x 50μm sections from frozen tissue blocks (in vial) |
€ 83,00 |
For profit organizations
The NBB operates a different pricing model for for-profit organizations and public-private partnerships; prices can be provided upon request via enbb@nin.knaw.nl.
Public private partnerships are defined as projects submitted by the academic partner, where all or part of the project costs are carried by industry, whereas the project execution lies predominantly within academia. As such the NBB remains accessible to academic researchers, while recognizing the for-profit character and interests of the funding source.
Invoice Information
An invoice for the supplied samples will be sent after the samples have been received by the applicant. If the NBB has not supplied samples to the applicant's organization before, 25% of the total financial contribution shall be invoiced in advance.
Please read more about our invoicing information and payment conditions here.
Material Transfer Agreement
The NBB requires a signed Material Transfer Agreement (MTA) to ensure that all conditions for the transfer and the use of the material are agreed upon by the receiving and providing parties. The MTA must be signed by an authorized representative of the university or institute in question. One master MTA per research institute (legal entity) covers all legal terms of delivery. Based on this MTA, all researchers in the research institute may apply for tissue.
After approval of an application, a Material Transfer Statement (MTS; a clear summary of the MTA), is sent to the researcher along with an Implementing Letter. This letter of approval specifies the material that will be supplied and the financial contribution. Upon receipt of the Implementing Letter and MTS, both signed by the applicant, the material will be supplied.
Summary of the material transfer process
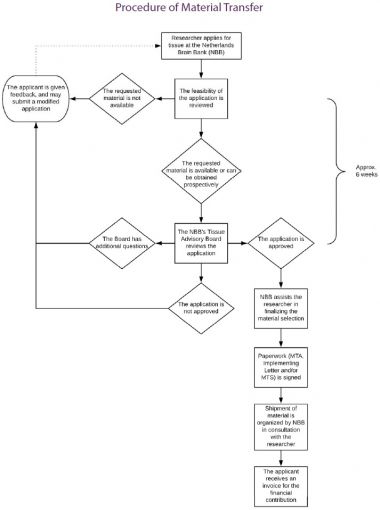
- The researcher employed by or affiliated with a certain Legal Entity, such as a university or a pharmaceutical company, sends an Application Form to the NBB.
- The Application Form is reviewed by the scientific committee of the NBB.
- When the Application is approved, the Researcher (‘Applicant’) is asked to provide the standard MTA to the person authorized to sign documents on behalf of the Legal Entity (e.g. board of directors), provided that no MTA has already been signed with that same Legal Entity. Formally the Legal Entity (called Recipient in the MTA) - not the individual researcher - is a party to the legal agreement. The responsibility to ensure compliance with the provisions of the MTA lies with the Legal Entity.
- When the Legal Entity has signed the MTA, details concerning Material Transfer (such as the amount of tissue provided, preservation mode and financial contribution) are specified in the Implementing Letter.
- All subsequent transfers to the Legal Entity only require signing of the Implementing Letter and optionally an MTS by the requesting scientist. Every Implementing Letter becomes an appendix to the existing MTA. Provisions of the framework MTA apply to every transfer within the same Legal Entity.
For more information on the material transfer procedure of the NBB, please read our brochure for tissue applicants.
Transport
Transportation of samples
The main applicant shall either collect the tissue samples at the address of the NBB or request the NBB to post the material to the recipient’s address by a courier of choice. When the brain samples will be shipped, the NBB will take all reasonable precautions to ensure proper packaging of the material for transportation purposes.
Transfer of data
The main applicant then also receives the extensive (pseudononymized) clinical and neuropathological information of the donors from which samples have been supplied. The clinical summary contains, among other data, the cause of death and the medical history (e.g. clinical course and medication use).
HomeBrain tissueApplication
